Annexin V-iFluor® 350 conjugate
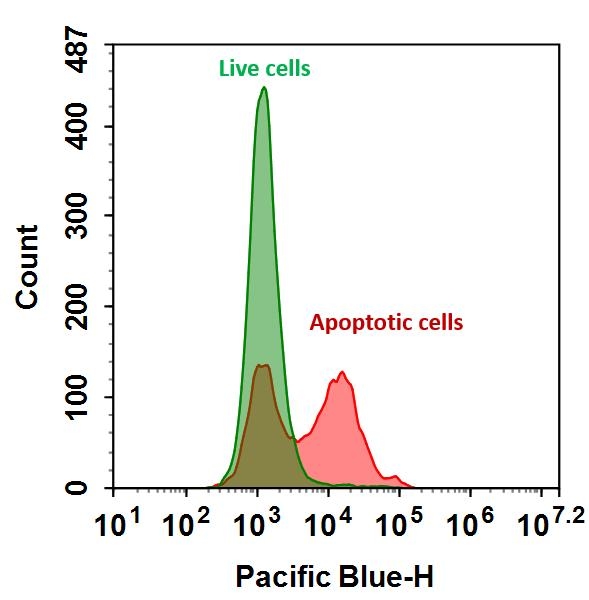
Annexins are a family of proteins that bind to phospholipid membranes in the presence of calcium. Annexin V is a valuable tool for studying cell apoptosis. It is used as a probe to detect cells expressing phosphatidylserine on the cell surface, a feature found in apoptosis and other forms of cell death. There are a variety of parameters that can be used for monitoring cell viability. Annexin V-dye conjugates are widely used to monitor cell apoptosis by measuring the translocation of phosphatidylserine (PS). In apoptosis, PS is transferred to the outer leaflet of the plasma membrane. The appearance of phosphatidylserine on the cell surface is a universal indicator of the initial/intermediate stages of cell apoptosis and can be detected before morphological changes can be observed. This fluorescent Annexin V conjugate has spectral properties similar to Alexa Fluor® 350 (Alexa Fluor® 350 is the trademark of Invitrogen).

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 20070 | 100 tests | Price |
Physical properties
| Molecular weight | ~36000 |
| Solvent | Water |
Spectral properties
| Correction factor (260 nm) | 0.83 |
| Correction factor (280 nm) | 0.23 |
| Extinction coefficient (cm -1 M -1) | 20000 1 |
| Excitation (nm) | 345 |
| Emission (nm) | 450 |
| Quantum yield | 0.95 1 |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| Storage | Freeze (< -15 °C); Minimize light exposure |
| UNSPSC | 12352200 |
Instrument settings
| Flow cytometer | |
| Excitation | 355 or 405 nm laser |
| Emission | 450/40 nm filter |
| Instrument specification(s) | Pacific Blue channel |
| Fluorescence microscope | |
| Excitation | DAPI filter set |
| Emission | DAPI filter set |
| Recommended plate | Black wall/clear bottom |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on October 8, 2024

