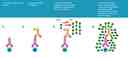
iFluor® 647 Styramide *Superior Replacement for Alexa Fluor 647 tyramide*
Example protocol
AT A GLANCE
- Fix/permeabilize/block cells or tissue
- Add primary antibody in blocking buffer
- Add HRP-conjugated secondary antibody
- Prepare Styramide™ working solution and apply in cells or tissue for 5-10 minutes at room temperature
PREPARATION OF STOCK SOLUTIONS
Unless otherwise noted, all unused stock solutions should be divided into single-use aliquots and stored at -20 °C after preparation. Avoid repeated freeze-thaw cycles
Add 100 µL of DMSO into the vial of iFluor® dye-labeled Styramide™ conjugate to make 100X Styramide™ stock solution.
Note: Make single-use aliquots, and store unused 100X stock solution at < -15 °C in a dark place and avoid repeat freeze-thaw cycles.
Add 10 µL of 3% hydrogen peroxide (not provided) to 90 µL of ddH2O.
Note: Prepare the 100X H2O2 solution fresh on the day of use.
PREPARATION OF WORKING SOLUTION
Every 1 mL of Reaction Buffer requires 10 µL of Styramide™ stock solution and 10 µL of H2O2 stock solution.
Note: The Styramide™ provided is enough for 100 tests based on 100 µL of Styramide™ working solution needed per coverslip or per well in a 96-well microplate.
Note: The Styramide™ working solution must be used within 2 hours after preparation and avoid direct exposure to light.
Make appropriate concentration of secondary antibody-HRP working solution as per the manufacturer's recommendations.
SAMPLE EXPERIMENTAL PROTOCOL
This protocol is applicable for both cells and tissues staining.
- Fix the cells or tissue with 3.7% formaldehyde or paraformaldehyde, in PBS at room temperature for 20 minutes.
- Rinse the cells or tissue with PBS twice.
- Permeabilize the cells with 0.1% Triton X-100 solution for 1-5 minutes at room temperature.
- Rinse the cells or tissue with PBS twice.
Deparaffinize and dehydrate the tissue according to the standard IHC protocols. Perform antigen retrieval with the preferred specific solution/protocol as needed. A protocol can be found at:
https://www.aatbio.com/resources/guides/paraffin-embedded-tissue-immunohistochemistry-protocol.html
Optional: Quench endogenous peroxidase activity by incubating cell or tissue sample in peroxidase quenching solution (such as 3% hydrogen peroxide) for 10 minutes. Rinse with PBS twice at room temperature.
Optional: If using HRP-conjugated streptavidin, it is advisable to block endogenous biotins by biotin blocking buffer.
- Block with preferred blocking solution (such as PBS with 1% BSA) for 30 minutes at 4 °C.
- Remove blocking solution and add primary antibody diluted in recommended antibody diluent for 60 minutes at room temperature or overnight at 4 °C.
- Wash with PBS three times for 5 minutes each.
Apply 100 µL of secondary antibody-HRP working solution to each sample and incubate for 60 minutes at room temperature.
Note: Incubation time and concentration can be varied depending on the signal intensity.
- Wash with PBS three times for 5 minutes each.
Prepare and apply 100 µL of Styramide™ working solution to each sample and incubate for 5-10 minutes at room temperature.
Note: If you observe a non-specific signal, you can shorten the incubation time with Styramide. You should optimize the incubation period using positive and negative control samples at various incubation time points. Or you can use a lower concentration of Styramide in the working solution.
- Rinse with PBS three times.
- Counterstain the cell or tissue samples as needed. AAT provides a series of nucleus counterstain reagents as listed in Table 1. Follow the instruction provided with the reagents.
Mount the coverslip using a mounting medium with anti-fading properties.
Note: To ensure optimal results, it is recommended to use either ReadiUse™ microscope mounting solution (Cat. 20009) or FluoroQuest™ TSA/PSA Antifade Mounting Medium *Optimized for Tyramide and Styramide Imaging* (Cat. 44890) instead of Vectashield® mounting media. There are instances where Vectashield® mounting media may not be suitable for certain TSA/PSA conjugates.
- Use the appropriate filter set to visualize the signal from the Styramide labeling.
Table 1. Products recommended for nucleus counterstain.
| Cat# | Product Name | Ex/Em (nm) |
| 17548 | Nuclear Blue™ DCS1 | 350/461 |
| 17550 | Nuclear Green™ DCS1 | 503/526 |
| 17551 | Nuclear Orange™ DCS1 | 528/576 |
| 17552 | Nuclear Red™ DCS1 | 642/660 |
Calculators
Common stock solution preparation
| 0.1 mg | 0.5 mg | 1 mg | 5 mg | 10 mg | |
| 1 mM | 81.193 µL | 405.966 µL | 811.932 µL | 4.06 mL | 8.119 mL |
| 5 mM | 16.239 µL | 81.193 µL | 162.386 µL | 811.932 µL | 1.624 mL |
| 10 mM | 8.119 µL | 40.597 µL | 81.193 µL | 405.966 µL | 811.932 µL |
Molarity calculator
| Mass (Calculate) | Molecular weight | Volume (Calculate) | Concentration (Calculate) | Moles | ||||
| / | = | x | = |
Spectrum
Product family
| Name | Excitation (nm) | Emission (nm) | Extinction coefficient (cm -1 M -1) | Quantum yield | Correction Factor (260 nm) | Correction Factor (280 nm) |
| iFluor® 350 Styramide *Superior Replacement for Alexa Fluor 350 tyramide* | 345 | 450 | 200001 | 0.951 | 0.83 | 0.23 |
| iFluor® 405 Styramide | 403 | 427 | 370001 | 0.911 | 0.48 | 0.77 |
| iFluor® 440 Styramide | 434 | 480 | 400001 | 0.671 | 0.352 | 0.229 |
| iFluor® 450 Styramide *Superior Replacement for Opal Polaris 480* | 451 | 502 | 400001 | 0.821 | 0.45 | 0.27 |
| iFluor® 460 Styramide | 468 | 493 | 800001 | ~0.81 | 0.98 | 0.46 |
| iFluor® 488 Styramide *Superior Replacement for Alexa Fluor 488 tyramide and Opal 520* | 491 | 516 | 750001 | 0.91 | 0.21 | 0.11 |
| iFluor® 514 Styramide *Superior Replacement for Opal 540* | 511 | 527 | 750001 | 0.831 | 0.265 | 0.116 |
| iFluor® 532 Styramide | 537 | 560 | 900001 | 0.681 | 0.26 | 0.16 |
| iFluor® 546 Styramide *Superior Replacement for Alexa Fluor 546 tyramide* | 541 | 557 | 1000001 | 0.671 | 0.25 | 0.15 |
Show More (22) | ||||||
Citations
Authors: Mustary, Umme Habiba and Maeno, Akiteru and Rahaman, Md Mostafizur and Ali, Md Hasan and Tokumoto, Toshinobu
Journal: Scientific Reports (2024): 24354
Authors: Wang, Huiru and Feng, Shanglong and Zhu, Yanliang and Zhang, Yafeng and Zhou, Ziwei and Nian, Zhigang and Lu, Xueqin and Peng, Peng and Wu, Shu and Zhou, Li
Journal: Blood Transfusion (2024)
Authors: Mohale, Mamello and Gundampati, Ravi Kumar and Kumar, Thallapuranam Krishnaswamy Suresh and Heyes, Colin D
Journal: Analytical biochemistry (2022): 114524
Authors: Ji, Tengfei and Ma, Keqiang and Wu, Hongsheng and Cao, Tiansheng
Journal: (2021)
Authors: Masibag, Angelique N and Bergin, Christopher J and Haebe, Joshua R and Zouggar, A{\"\i}cha and Shah, Muhammad S and Sandouka, Tamara and da Silva, Amanda Mendes and Desrochers, Fran{\c{c}}ois M and Fournier-Morin, Aube and Benoit, Yannick D
Journal: Iscience (2021): 103442