Propidium Iodide Applications & Common Issues
Propidium iodide is a small, aromatic compound that is commonly used in research and development as a fluorescent nucleic acid binding stain. PI is often used to differentiate between apoptotic and necrotic cells from healthy cells as it freely penetrates cell membranes of dead or dying cells but is excluded from viable cells. Propidium iodide stains nucleic acids, like DNA and RNA, and can therefore be used as an indicator of membrane integrity in flow cytometry, in situ hybridization, and immunohistochemistry applications.
After addition of propidium iodide to a sample, cells with permanently or reversibly damaged cell membranes will fluoresce red. In this way, propidium iodide makes it possible to identify and quantitate dead and necrotic cells rapidly and reliably. Propidium iodide helps determine overall cell viability, which is an important parameter used to monitor the response of a cell population to cytotoxic drugs or other environmental factors.
Viability studies typically include a propidium iodide stain, coupled with the apoptosis marker stain (such as Annexin V-FITC or iFluor® 488 that has a bright green fluorescence). When used together, total cell counts can be obtained and the morphologies of cells within a sample may be observed. For example, intact cells (iFluor® 488-PI-), early apoptotic (iFluor® 488+PI-) and late apoptotic or necrotic cells (iFluor® 488+PI+) can all be discriminated by using these two stains in tandem.
After addition of propidium iodide to a sample, cells with permanently or reversibly damaged cell membranes will fluoresce red. In this way, propidium iodide makes it possible to identify and quantitate dead and necrotic cells rapidly and reliably. Propidium iodide helps determine overall cell viability, which is an important parameter used to monitor the response of a cell population to cytotoxic drugs or other environmental factors.
Viability studies typically include a propidium iodide stain, coupled with the apoptosis marker stain (such as Annexin V-FITC or iFluor® 488 that has a bright green fluorescence). When used together, total cell counts can be obtained and the morphologies of cells within a sample may be observed. For example, intact cells (iFluor® 488-PI-), early apoptotic (iFluor® 488+PI-) and late apoptotic or necrotic cells (iFluor® 488+PI+) can all be discriminated by using these two stains in tandem.
| Tools: | FAQs: |
Example PI Staining Protocol
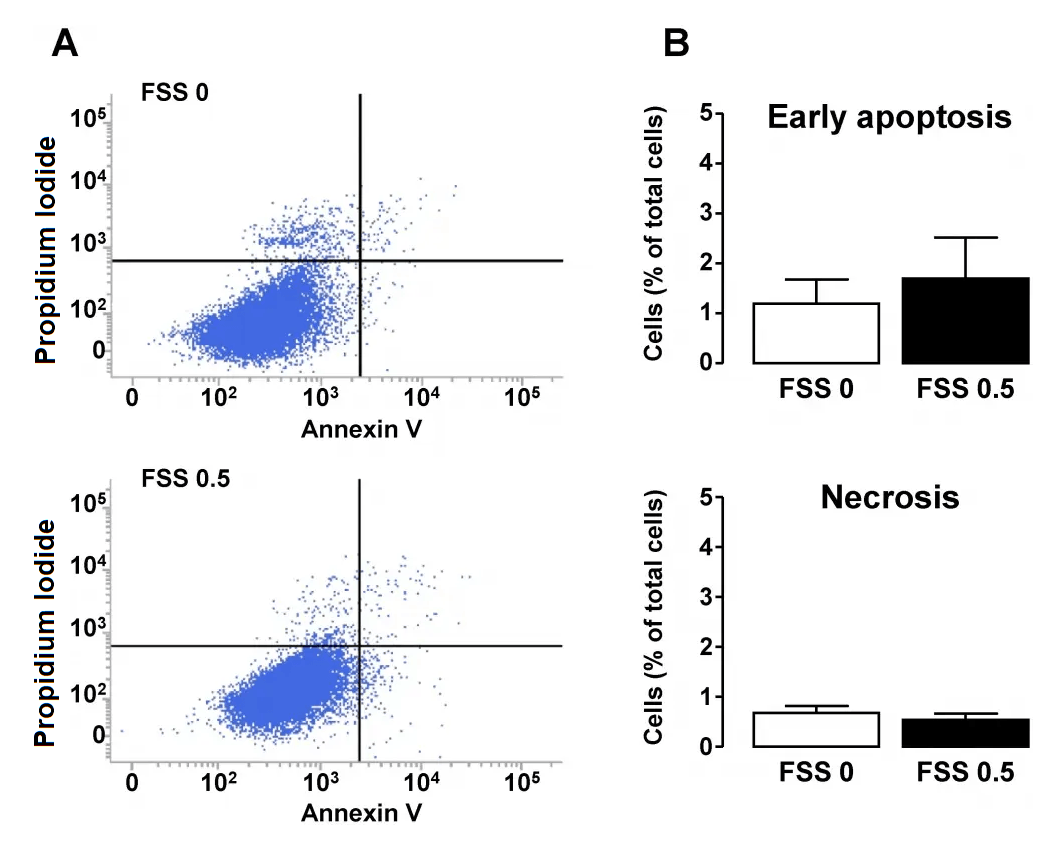
Effect of FSS on apoptosis and necrosis in tubular cells. Confluent monolayers of HK-2 cells were submitted to FSS 0 (static) or FSS 0.5 Pa (FSS 0.5) for 48h. A/ Cells were stained with Annexin-V and then immediately subjected to analysis of phosphatidylserine externalization (Annexin-V fluorescence, X-axis) and Propidium Iodide (PI) uptake (PI fluorescence, Y-axis) using flow cytometry. Living, early apoptotic or necrotic (primary or secondary) cells were distinguished by the criteria of Annexin-V-/PI-(bottom left quadrant), Annexin-V+/PI- (bottom right quadrant) and Annexin-V+/PI+ (upper right quadrant), respectively. B/ Proportions of early apoptosis and necrosis cells were quantified and results are expressed as a percentage of the total population of cells. Data represent mean ± SEM of 7 experiments. *HK-2 cells were assessed for apoptosis and necrosis using Cell Meter Annexin V Binding Apoptosis Assay Kit according to the manufacturer's instructions. Source: Shear Stress-Induced Alteration of Epithelial Organization in Human Renal Tubular Cells by Damien Maggiorani, et al., PLoS ONE, July 2015.
- Cell Preparation: Dissolve the PI into a buffered solution such as PBS or Tris-EDTA buffer. The concentration of PI in staining solution may vary depending on experimental conditions, but is typically 1-5 mg/mL.
- Cell Harvesting: Cells are detached from the growth surface (e.g. culture dish) by using trypsinization for adherent cells, or centrifugation for suspension cells. Aliquot up to 1X10^6 cells/100 μL into polystyrene tubes and add 70% ethanol while gently vortexing.
- Cell Washing: Wash the cells by adding 2mL PBS, centrifuging at 300 X g for 5 minutes and then pour out the buffer from pelleted cells. Repeat this step a total of 2 times to remove any residual media or serum components.
- Resuspension of cells: The staining solution containing PI (e.g. 0.1% BSA in PBS, RNase, PI stock solution) should then be added to the cell suspension to achieve desired concentration of PI. Gently mix the cells and PI solution to ensure even staining.
- Incubation of cells: Incubate the cells at 37 degrees Celsius for a period of time depending on the experiment. Cells are typically incubated with PI for greater than 30 minutes in a dark room to be protected from light degradation.
- Analyzing cells: Following incubation, PI stained cells can be analyzed using flow cytometry, and are excited by a laser emitting light at a wavelength of 488 nm (blue-green). The emitted fluorescence is red in color and at a wavelength of 617 nm. When used in IHC, PI is often used as a nuclear counterstain to visualize cellular nuclei within tissue sections.
This protocol should be adapted based on experimental requirements and optimization.
Propidium Iodide Advantages & Common Issues
The major advantage of using propidium iodide staining techniques is that these methods cause minimal cell loss and cell damage by hydrolysis can be avoided. There are, however, a few common issues with propidium iodide. Conventional Annexin V/PI protocols, as well as similar staining protocols, may lead to a significant number of false positive events (>40%). Such false positives are associated with how propidium iodide will also stain RNA within the cytoplasmic compartment. This issue can occur in both primary cells and cell lines and occurs most often with larger cells that have a smaller nuclear-to-cytoplasmic-ratio.
Since propidium iodide is a membrane impermeable DNA-binding stain, it is generally also used alongside a membrane-permeable DNA-binding counterstain in bacterial viability studies. However, in the research, propidium iodide staining of adherent cells in biofilms has shown to significantly underestimate the actual bacterial viability due to the presence of extracellular nucleic acids (eNA). Upon observation, it appears that a false dead layer of red, propidium iodide-stained cells will appear. This occurrence can sometimes cover up a subpopulation of double-stained cells that have green interiors, indicating viability, under a red layer which hints at DNA being stained outside of intact membranes.
| Assaywise Letter: |
Therefore, viability staining results of such cell populations should always be validated by an alternative method for estimating viability, e.g., confocal laser scanning microscopy (CLSM) or fluorescence microscopy. The use of propidium iodide, in most cases, is limited to fixed or permeabilized cells to allow the dye to enter into the cells that would be otherwise actively pumped out of a living cell. In permeabilization and fixation, alcohol or aldehydes are usually choice reagents. Importantly, aldehyde and alcohol are often incompatible with fluorescent proteins and some surface markers, so paraformaldehyde may be a more appropriate solution. As far as safety goes, propidium iodide is a suspected carcinogen, and may cause skin, eye, and respiratory irritation.
Products
Table 1. Cell Meter™ Annexin V Binding Apoptosis Assay Selection Guide
| Annexin V Conjugate ▲ ▼ | Dead Cell Stain ▲ ▼ | Annexin V Conjugate Ex/Em ▲ ▼ | Dead Cell stain Ex/Em ▲ ▼ | Unit Size ▲ ▼ | Cat No. ▲ ▼ |
| Annexin V-iFluor® 488 | PI | 490/ 520 nm | 534/617 nm | 100 Tests | 22824 |
| Annexin V-iFluor® 555 | None | 554/578 nm | *** | 100 Tests | 22825 |
| Annexin V-iFluor® 594 | None | 590/610 nm | *** | 100 Tests | 22826 |
| Annexin V-iFluor® 647 | None | 650/668 nm | *** | 100 Tests | 22827 |
| Annexin V-mFluor™ Violet 450 | PI | 405/450 nm | 534/617 nm | 100 Tests | 22828 |
| Annexin V-mFluor™ Violet 500 | PI | 414/508 nm | 534/617 nm | 100 Tests | 22829 |
| Annexin V-mFluor™ Violet 550 | PI | 424/560 nm | 534/617 nm | 100 Tests | 22830 |
| Annexin V-APC | PI | 651/662 nm | 534/617 nm | 100 Tests | 22837 |
| Annexin V-PE | Nuclear Red™ DCS1 | 565/575 nm | 631/651 nm | 100 Tests | 22838 |
| Annexin V-FITC | PI | 490/520 nm | 534/617 nm | 100 Tests | 22839 |
Table 2. Membrane impermeant nucleic acid stains for labeling the nucleus in dead and fixed cells.
| Product name ▲ ▼ | Cell Permeability ▲ ▼ | Live/Fixed Cells ▲ ▼ | Ex/Em (nm) ▲ ▼ | Filter Set ▲ ▼ | Unit Size ▲ ▼ | Cat No. ▲ ▼ |
| Nuclear Blue™ DCS1 | Membrane impermeant | Dead and fixed cells | 344/465 | DAPI | 0.5 mL | 17548 |
| DiYO™-1 [equivalent to YOYO®-1] | Membrane impermeant | Dead and fixed cells | 491/508 | FITC | 1 mg | 17575 |
| Nuclear Green™ DCS1 | Membrane impermeant | Dead and fixed cells | 501/526 | FITC | 0.5 mL | 17550 |
| Nuclear Green™ Photo-Fixable DCS1 *5 mM DMSO Solution* | Membrane impermeant | Dead, fixed, & apoptotic cells | 502/527 | FITC | 1 mg | 17570 |
| DiTO™-1 [equivalent to TOTO®-1] | Membrane impermeant | Dead and fixed cells | 514/531 | FITC | 0.2 mL | 17575 |
| TWO-PRO™ 1 [equivalent to TO-PRO®-1] | Membrane impermeant | Dead and fixed cells | 515/531 | FITC | 0.2 mL | 17571 |
| Nuclear Orange™ DCS1 | Membrane impermeant | Dead and fixed cells | 512/554 | TRITC | 0.5 mL | 17551 |
| Propidium Iodide *CAS 25535-16-4* | Membrane impermeant | Dead and fixed cells | 537/618 | Texas Red | 5 g | 17516 |
| Propidium Iodide *10 mM aqueous solution* | Membrane impermeant | Dead and fixed cells | 537/618 | Texas Red | 1 mL | 17517 |
| DiYO™-3 [equivalent to YO-YO®-3] | Membrane impermeant | Dead and fixed cells | 612/631 | Cy5 | 0.2 mL | 17580 |
Table 3. Selection guide for Live or Dead™ Cell Viability Assays for eukaryotic cells.
| Kit ▲ ▼ | Probes ▲ ▼ | Ex/Em (nm) ▲ ▼ | Em Colors ▲ ▼ | Applications ▲ ▼ | Unit Size ▲ ▼ | Cat No. ▲ ▼ |
| Live or Dead™ Cell Viability Assay Kit *Green/Red Dual Fluorescence* | CytoCalcein™ Green Propidium Iodide | 494/514 537/618 | Green (Live) Red (Dead) | FC, FM or MA¹ | 200 Tests | 22789 |
| Live or Dead™ Cell Viability Assay Kit *Green/Red Dual Fluorescence* | CytoCalcein™ Green Propidium Iodide | 494/514 537/618 | Green (Live) Red (Dead) | FC, FM or MA¹ | 1000 Tests | 22760 |
| Live or Dead™ Cell Viability Assay Kit *Red/Blue Dual Fluorescence* | Cellbrite™ Red Nuclear Blue™ DCS1 | 613/631 348/469 | Red (Live) Blue (Dead) | FC, FM or MA¹ | 200 Tests | 22788 |
Table 4. MycoLight™ Fluorescence Live/Dead Bacterial Imaging Kits for bacteria.
| Kit ▲ ▼ | Probes ▲ ▼ | Ex/Em (nm) ▲ ▼ | Em Colors ▲ ▼ | Applications ▲ ▼ | Unit Size ▲ ▼ | Cat No. ▲ ▼ |
| MycoLight™ Fluorescence Live/Dead Bacterial Imaging Kit | MycoLight™ 520 Propidium Iodide | 488/530 537/618 | Green (Live) Red (Dead) | FM¹ | 100 Tests | 22789 |
References
Propidium Iodide
Propidium iodide staining underestimates viability of adherent bacterial cells
Conventional apoptosis assays using propidium iodide generate a significant number of false positives that prevent accurate assessment of cell death
Propidium iodide staining underestimates viability of adherent bacterial cells
Original created on February 15, 2024, last updated on February 15, 2024
Tagged under: propidium iodide, DNA, viability, dead cell stain
