Fluorescent in situ hybridization (FISH)
Overview
Fluorescent in situ hybridization (FISH) is a classic method of research that is used to locate the positions of specific DNA sequences on chromosomes. In FISH, in situ hybridization is coupled with fluorescent labeling where probes are labeled with an F-labeled nucleotide and then targeted to the DNA or gene or interest. In this coupled technology, dsDNA can be particularly identified and targeted and then visualized through a fluorescence microscope or other applicable imaging system.
FISH technology has been used extensively as a macromolecule recognition tool to effectively identify and map chromosomal, and sub-chromosomal, abnormalities during the annotation phase of the Human Genome Project. Now, FISH is used in research more so for its highly sensitive and specific capabilities, which may be used as a diagnostic tool for the identification of genetic and chromosomal disorders, principally directed for clinical diagnosis.
There are many slight variations in how FISH may be performed, however each procedure follows the same general principles. Firstly, a probe is constructed. The probe is then fluorescently labeled directly by a process called Nick translation. Next, the labeled probe and target DNA/RNA are denatured separately and then sequentially annealed to one another. Locations of the fluorescent probe within the target DNA/RNA can then be identified through various means of analysis. Below is an example FISH protocol using DNA from HeLa cells, using probes constructed through PCR and direct fluorescent labeling methods, along with hybridization and secondary washing steps.
Example Protocol
- Probe Creation using dPCR
Materials Specifications DNA extraction kit (Qiagen's FlexiGene DNA kit) used to obtain gDNA from HeLa cells Forward primers 5'-TTA GGG TTA GGG TTA GGG TTA GGG TTA GGG-3' Reverse primer 5'-CCC TAA CCC TAA CCC TAA CCC TAA CCC TAA-3' PCR kit Complete with enzyme, buffer, or master mixes Fluorescent labelers dUTPs or biotinylated dUTP (at a 1:1 ratio of iF488-dUTP:dTTP) dNTPs Used to provide nucleotides to unzipped strands during PCR PCR and gel clean up kit (Enzo Biosciences) used to purify DNA probes.
- Follow the steps on the DNA extraction kit to extract gDNA from HeLa cells, or use a manual in-house protocol if preferred.
- Using a spectrophotometer or absorbance microplate reader, ensure there is at least 100 ng of gDNA starting material.
- Combine 100 ng gDNA with appropriate amounts of PCR enzyme and PCR buffer.
Note: Consequently, a PCR master mix may be used that contains a DNA polymerase, dNTPs, MgCL2 and buffer. If using a PCR master mix, only primers, fluorescent probes, and water (DNase/RNase free, to make up the volume) need to be added to the working sample. - Add 500 uM dNTPs to the working sample.
- Add 300 uM of forward and 300 uM of the reverse primers to the working sample.
- Add an appropriate amount of dUTPs or biotinylated dUTP.
- Perform PCR as per the PCR enzyme's profile
- Purify the probes using the PCR and gel clean up kit.
- FISH protocol
Materials Specifications Colcemid 0.1 µg/mL, KaryoMAX Colcemid Solution (Thermo Fisher scientific, Cat; 15212021) KCl 0.075 M, prewarmed to 37 ℃ Fixative1 3:1, methanol:acetic acid, ice cold Culture medium As used for cell culture, prewarmed to 37 ℃ Transfer tubes 15 mL falcon tube, 1.5 mL Eppendorf tube Trypsin Commercially available 100% ETOH DI H1O Poly-L-Lysine Glass microscope slide - The fixative must always be freshly prepared.
- Day 1: Cell Treatment With Colcemid
- Bring HeLa cells to 70-80% confluency
- Harvested cells by trypsinization
- Add 1 mL of trypsin to the cells, and softly pipette the sample.
- Incubate at 37 ℃ for 3-5 mins.
- Add 5 mL of pre-warmed culture medium to the sample and transfer the sample to a 15 mL falcon tube.
- Add 0.1 µg/mL of Colcemid and incubate the sample at 37 ℃ for 4 hrs.
- After incubation, centrifuge the sample at 1700 rpm for 5 mins.
- After centrifugation, carefully remove the supernatant.
- Dissolve the pellet in 8 mL of 0.075 M KCl (pre-warm at 37 ℃)
- Incubate the sample for 10 mins at 37 ℃.
- After incubation, add 1 mL of ice cold fixative to the sample.
- Centrifuge the sample at 1700 rpm for 5 mins.
- After centrifugation, carefully remove the supernatant.
- Add 4 mL of ice cold fixative to the pellet.
- Incubate the sample at 4 ℃ for 10 mins.
- Inculcate the sample at 37 ℃ for 15 mins.
- After incubation, centrifuge the sample at 1700 rpm for 5 mins.
- After centrifugation, carefully remove the supernatant.
- Add 1 mL of fixative to the sample
- Transfer the sample to 1.5 mL Eppendorf tube.
- Centrifuged the sample at 2300 rpm for 5 mins. After centrifugation, carefully remove the supernatant.
- Add fixative (enough to cover and resuspend cells) to the pellet and store at -20 ℃.
- Slide Preparation
- Wash slides with 100% ETOH and DI H2O.
- Incubate slides with 50 µg/mL of poly-L-Lysine at room temperature.
Note: Pre-prepared poly-L-Lysine slides are also commercially available.
- Day 2: Metaphase Spread
Materials Specifications DI H2O Commercially available NP-40 Lysis Buffer Diluted to 0.1% using 2X SSC Buffer, commercially available Acetic acid 45%, Commercially available Poly-L-Lysine slides Previously prepared DNA probes 20X SSC Buffer To make 100 mL solution:
- Combine 17.532 g NaCl and 8.823 g Sodium citrate.
- Dissolved in 100 mL distilled water, and adjust to pH 7.
ETOH 70% and 100% Denaturation buffer Prewarmed to 80 ℃, 70 % of total volume formamide with 2X SSC, pH 7. Sperm DNA To make single stranded DNA:
- Boil aliquot amount of stock DNA at 100 ℃ for 5 mins.
- Put the sample on ice for 5 mins.
- Store the sample at -20 ℃ until use in the hybridization buffer.
Hybridization buffer Includes concentrations by volume:
- 55 % formamide
- 2X SSC
- 11% dextran sulfate
- 0.11% SDS
- 220 ug/ml sperm DNA
- The next day, wash the slides with DI H2O.
- Wash the slides with 45% acetic acid.
- Adhere cells to the slides
- Cells in fixative that are stored in -20 ℃ should be centrifuged 1-2 times before
- Dropping on slides.
- Hold the slides at a 45° angle.
- At a 3-5 cm height, apply 1 drop of cells (~15 uL from a 200-300 uL cell suspension) onto the slides.
- Allow the slides to air dry for 5 mins.
- Incubate the slides at 37 ℃ for 1 hr.
- Cell Pretreatment and Denaturation
- Incubate prepared slides with enough 0.1 % NP-40 in 2X SSC to cover the sample for 30 mins at 37 ℃.
- Add 70% ethanol to prepared slides and allow them to air dry for 3-5 mins.
- Add 100% ethanol to prepared slides and allow them to air dry for 3-5 mins.
- Add 20-40 uL of pre-warmed denaturation buffer to the prepared slides, and a cover slip to the top.
- Incubated the prepared slides at 80 ℃ for 5 mins.
- Remove the cover slip by dipping prepared slides in 70% ethanol
- Add 100 % ethanol to prepared slides and allow them to air dry for 2 mins.
- Probe Preparation
- Add 1 uL of the probe, previously made, to 9 uL of hybridization buffer. Vortex well.
- Incubated the solution at 37 ℃ for 10 mins.
- Denatured the solution at 80 ℃ for 5 mins in a thermal cycler.
- Place the solution on ice until applied to the prepared slides.
- Hybridization:
- Add 10 uL of the solution to the, now denatured, slides. Place a cover slip on top.
- Seal the slides with rubber cement. Incubate the slides at 45-50 ℃ for ~5 mins.
- Incubated the sealed slides at 37 ℃ until the next day. If necessary, place a wet paper towel under the slides to help maintain moisture.
- Day-3: Post-Hybridization Wash and DAPI staining:
Materials Specifications 2X SSC buffer Previously prepared Hybridized slides NP-40 Lysis Buffer Diluted to 0.3% in 0.4X SSC Buffer, commercially available 2X SEC Previously prepared DAPI Mounting Medium To prepare:
Dilute 10mM DAPI in mounting medium until the concentration of 5uM is reached
- Remove the coverslip by placing the slides in a 2X SSC buffer.
- Wash the slides with 0.3% NP-40 in 0.4X SSC buffer for 2 mins at 73-75 ℃.
- Wash the slides with 2X SSC Buffer for 1 min at room temperature.
- Allow the slides to air dry. Mount the cover slip by applying DAPI Mounting Medium.
- Images can be acquired using a fluorescence microscope at 20X magnification.
Visualization and Analysis
The power of FISH can be amplified by the use of multiple fluorescent colors in tandem. Multicolor FISH can be used to identify as many labeled features as there are differing fluorophores used in the hybridization process. Single colors, as well as various combinations of colors, can be simultaneously detected.
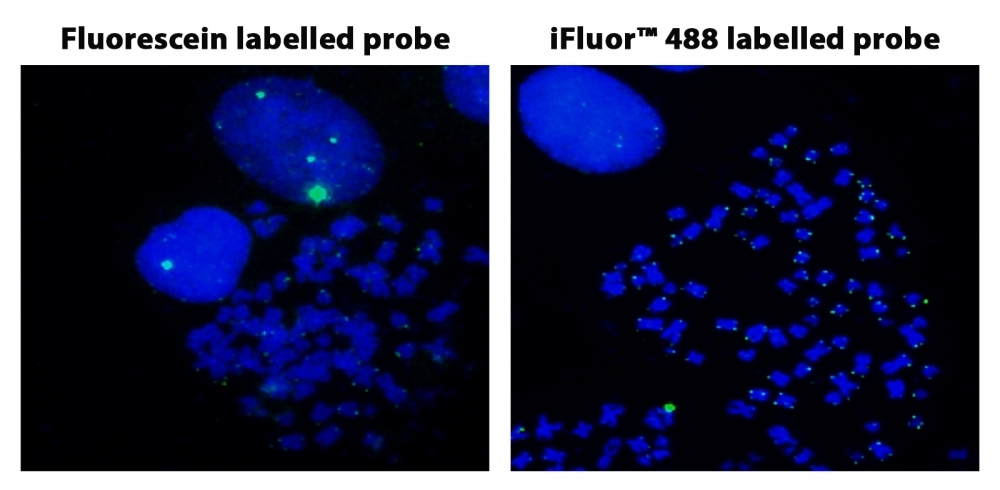
Fluorescence In Situ Hybridization of Fluorescein and iFluor® 488-dUTP labelled Telomere probes in metaphase HeLa cells.
Epifluorescent microscopy is commonly used for this multicolor analysis, and similar to other fluorescence microscopes, the excitation light from the light source is passed through a filter before reaching the objective lens and dichroic mirror. This excitation filter allows a single band of a particular wavelength through, dependent on the peak fluorescence excitation wavelength of the fluorophore. The excitation filter can be in the form of filter cubes or a filter wheel with multipass dichroic and barrier filters. The produced signals must be isolated by an emission filter that ensures that only the wavelength emitted from the targeted fluorophore in the sample passes through.
Traditionally, data was collected through digital charged coupled devices (CCDs), high-speed color film, or intensified video cameras, though these methods had issues with color fidelity and there existed problems when processing superimposed images, with different colors, within a single specimen.
Recently, much development has been made to overcome these limitations with the creation of quantitative imaging analysis software suites. These analysis programs are capable of capturing images across multiple wavelengths and/or focal planes, with the ability to retrieve and store large volumes of multicolor image data sets. The software offer the ability to focus at multiple wavelengths, record the slide locations of cells, quantitatively measure probe counts and/or intensity, and determine cell morphology. It may also be able to analyze multicolor metaphase chromosomes, map genes, offer comparative genomic hybridization ratios, as well as determine karyotype generation.
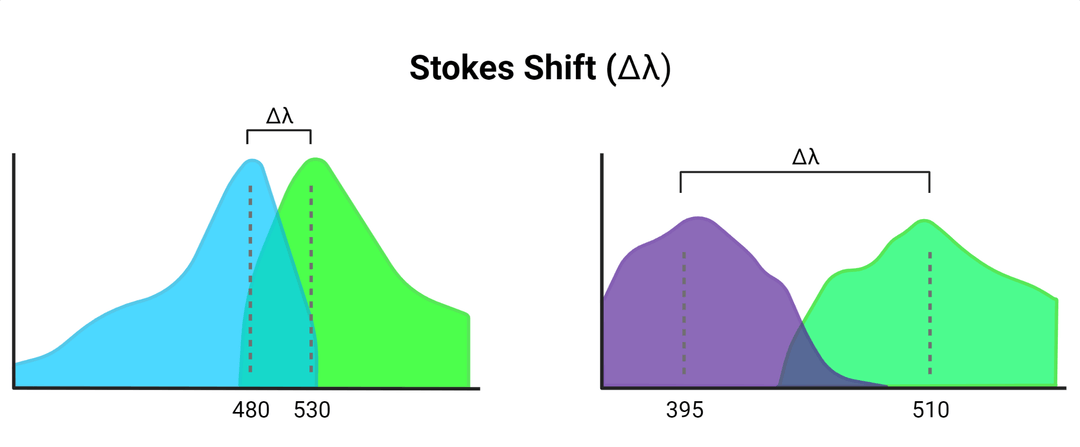
Confocal laser scanning microscopy may also be used for visualization. This technique uses light emitted by a laser, at a specific wavelength to maximally excite the fluorophore, which it will then emit at a somewhat longer wavelength. The difference between these two wavelengths is known as the Stokes shift.
The light runs through a pinhole aperture before hitting the dichromatic mirror directed towards the specimen, where secondary fluorescence is then emitted. Instead of illuminating the entire sample at once, the laser light only illuminates a given spot at a specified depth. Because of this, out-of-focus signals from features located above and/or below the focal plane contribute to the high signal-to-noise ratio of images produced. As the detector pinhole required for focusing images also may reduce signal strength, confocal laser scanning microscopy typically requires higher fluorescence signal yield to be effective.
Considerations and Limitations
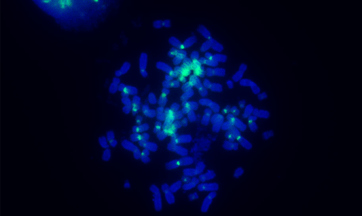
Pan-centromeric staining via in situ hybridization using a biotinylated PNA probe. Jurkat cells were fixed and permeabilized using a standard protocol. The centromere of each chromosome was detected with streptavidin HRP conjugate and visualized with iFluor® 488 Styramide reagent. Chromosomes were counterstained with DAPI.
In many cases, it may be necessary to multiplex spectral techniques to increase the number of detectable targets. Many experimental steps require empirical optimization specifically for the samples and probes used, which may prove time consuming and costly. Long hybridization times are essential to ensure the appropriate saturation of signals throughout the specimen, so FISH is also an inherently lengthy process and requires experienced personnel, though advances in assay automation may alleviate these limitations in the future.
Though many variations of FISH exist, a major downfall of this technique is that it can only detect known genetic aberrations. FISH can be used only to confirm previously characterized imbalances and abnormalities, and cannot serve as a screening test for novel chromosomal rearrangements.
Product Ordering Information
Table 1. ReadiLink™ FISH Fluorescence Imaging Kits
| Cat# ▲ ▼ | Product Name ▲ ▼ | Unit Size ▲ ▼ |
| 17310 | ReadiLink™ iFluor® 488 FISH Fluorescence Imaging Kit | 25 Reactions |
| 17313 | ReadiLink™ iFluor® 555 FISH Fluorescence Imaging Kit | 25 Tests |
| 17316 | ReadiLink™ iFluor® 647 FISH Fluorescence Imaging Kit | 25 Tests |
Table 2. Styramide™ Signal Amplification Kits and Standalone Dye-Labeled Styramide™ Substrates for ICC, IHC and FISH
| Cat# ▲ ▼ | Product Name ▲ ▼ | Ex (nm) ▲ ▼ | Em (nm) ▲ ▼ | Size ▲ ▼ |
| 45000 | iFluor® 350 Styramide *Superior Replacement for Alexa Fluor 350 tyramide* | 345 | 442 | 100 Slides |
| 45020 | iFluor® 488 Styramide *Superior Replacement for Alexa Fluor 488 tyramide* | 491 | 514 | 100 Slides |
| 45025 | iFluor® 546 Styramide *Superior Replacement for Alexa Fluor 546 tyramide* | 541 | 557 | 100 Slides |
| 45027 | iFluor® 555 Styramide *Superior Replacement for Alexa Fluor 555 tyramide* | 552 | 567 | 100 Slides |
| 45030 | iFluor® 568 Styramide *Superior Replacement for Alexa Fluor 568 tyramide* | 568 | 587 | 100 Slides |
| 45035 | iFluor® 594 Styramide *Superior Replacement for Alexa Fluor 594 tyramide* | 592 | 619 | 100 Slides |
| 45045 | iFluor® 647 Styramide *Superior Replacement for Alexa Fluor 647 tyramide* | 649 | 665 | 100 Slides |
| 45050 | iFluor® 680 Styramide *Superior Replacement for Alexa Fluor 680 tyramide* | 676 | 695 | 100 Slides |
| 45055 | iFluor® 700 Styramide *Superior Replacement for Alexa Fluor 700 tyramide* | 685 | 710 | 100 Slides |
| 45065 | iFluor® 750 Styramide *Superior Replacement for Alexa Fluor 750 tyramide* | 749 | 775 | 100 Slides |
.png&w=1200&q=100)