iFluor® 597 succinimidyl ester
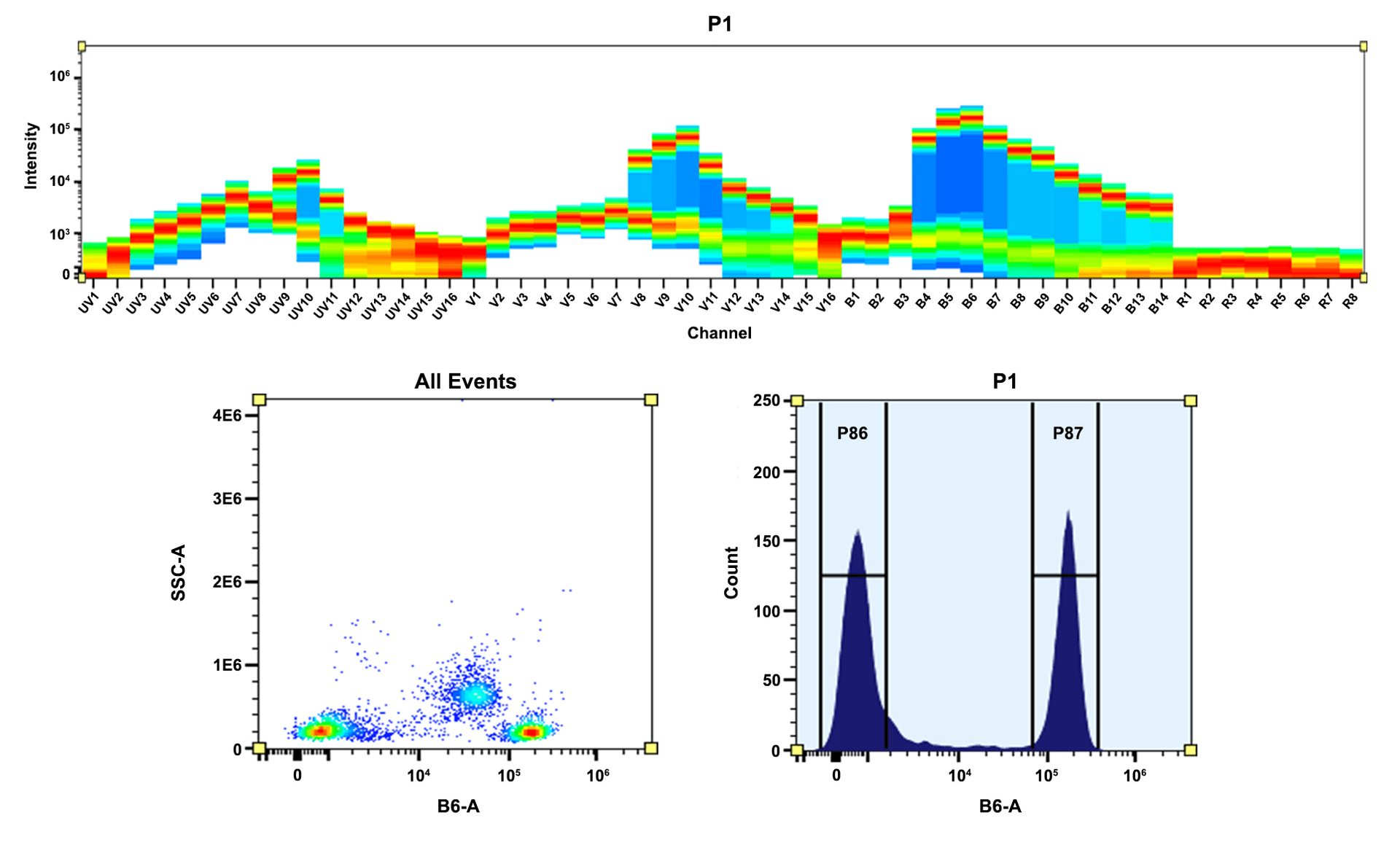
AAT Bioquest's iFluor® dyes are optimized for labeling proteins, particularly antibodies. These dyes are bright, photostable, and have minimal quenching on proteins. They can be well excited by the major laser lines of fluorescence instruments (e.g., 350, 405, 488, 555, 633, 638, 647, 660, and 802 nm). iFluor® 597 is a unique color for fluorescence imaging and flow cytometry applications. iFluor® 597 is an excellent acceptor dye for preparing PE-tandem dyes. These iFluor® 597 tandem colors offer a set of unique color profiles for spectral flow cytometry. Compared to Alexa Fluor® 594 tandems, iFluor® 597 tandems have improved FRET efficiency and photostability.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 1050 | 1 mg | Price |
Physical properties
| Molecular weight | 1058.29 |
| Solvent | DMSO |
Spectral properties
| Absorbance (nm) | 597 |
| Correction factor (260 nm) | 0.335 |
| Correction factor (280 nm) | 0.514 |
| Extinction coefficient (cm -1 M -1) | 100000 1 |
| Excitation (nm) | 598 |
| Emission (nm) | 618 |
| Quantum yield | 0.7 1 |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| Storage | Freeze (< -15 °C); Minimize light exposure |
| UNSPSC | 12171501 |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on March 9, 2026

