iFluor® 597 succinimidyl ester
Example protocol
PREPARATION OF STOCK SOLUTIONS
Unless otherwise noted, all unused stock solutions should be divided into single-use aliquots and stored at -20 °C after preparation. Avoid repeated freeze-thaw cycles
Mix 100 µL of a reaction buffer (e.g., 1 M sodium carbonate solution or 1 M phosphate buffer with pH ~9.0) with 900 µL of the target protein solution (e.g. antibody, protein concentration >2 mg/mL if possible) to give 1 mL protein labeling stock solution.
Note: The pH of the protein solution (Solution A) should be 8.5 ± 0.5. If the pH of the protein solution is lower than 8.0, adjust the pH to the range of 8.0-9.0 using 1 M sodium bicarbonate solution or 1 M pH 9.0 phosphate buffer.
Note: The protein should be dissolved in 1X phosphate buffered saline (PBS), pH 7.2-7.4. If the protein is dissolved in Tris or glycine buffer, it must be dialyzed against 1X PBS, pH 7.2-7.4, to remove free amines or ammonium salts (such as ammonium sulfate and ammonium acetate) that are widely used for protein precipitation.
Note: Impure antibodies or antibodies stabilized with bovine serum albumin (BSA) or gelatin will not be labeled well. The presence of sodium azide or thimerosal might also interfere with the conjugation reaction. Sodium azide or thimerosal can be removed by dialysis or spin column for optimal labeling results.
Note: The conjugation efficiency is significantly reduced if the protein concentration is less than 2 mg/mL. For optimal labeling efficiency the final protein concentration range of 2-10 mg/mL is recommended.
Add anhydrous DMSO into the vial of iFluor™ 597 SE to make a 10 mM stock solution. Mix well by pipetting or vortex.
Note: Prepare the dye stock solution (Solution B) before starting the conjugation. Use promptly. Extended storage of the dye stock solution may reduce the dye activity. Solution B can be stored in freezer for two weeks when kept from light and moisture. Avoid freeze-thaw cycles.
SAMPLE EXPERIMENTAL PROTOCOL
This labeling protocol was developed for the conjugate of Goat anti-mouse IgG with iFluor™ 597 SE. You might need further optimization for your particular proteins. Each protein requires distinct dye/protein ratio, which also depends on the properties of dyes. Over labeling of a protein could detrimentally affects its binding affinity while the protein conjugates of low dye/protein ratio gives reduced sensitivity.
Use 10:1 molar ratio of Solution B (dye)/Solution A (protein) as the starting point: Add 5 µL of the dye stock solution (Solution B, assuming the dye stock solution is 10 mM) into the vial of the protein solution (95 µL of Solution A) with effective shaking. The concentration of the protein is ~0.05 mM assuming the protein concentration is 10 mg/mL and the molecular weight of the protein is ~200KD.
Note: We recommend to use 10:1 molar ratio of Solution B (dye)/Solution A (protein). If it is too less or too high, determine the optimal dye/protein ratio at 5:1, 15:1 and 20:1 respectively.
- Continue to rotate or shake the reaction mixture at room temperature for 30-60 minutes.
The following protocol is an example of dye-protein conjugate purification by using a Sephadex G-25 column.
- Prepare Sephadex G-25 column according to the manufacture instruction.
- Load the reaction mixture (From "Run conjugation reaction") to the top of the Sephadex G-25 column.
- Add PBS (pH 7.2-7.4) as soon as the sample runs just below the top resin surface.
Add more PBS (pH 7.2-7.4) to the desired sample to complete the column purification. Combine the fractions that contain the desired dye-protein conjugate.
Note: For immediate use, the dye-protein conjugate need be diluted with staining buffer, and aliquoted for multiple uses.
Note: For longer term storage, dye-protein conjugate solution need be concentrated or freeze dried.
Characterize the Desired Dye-Protein Conjugate
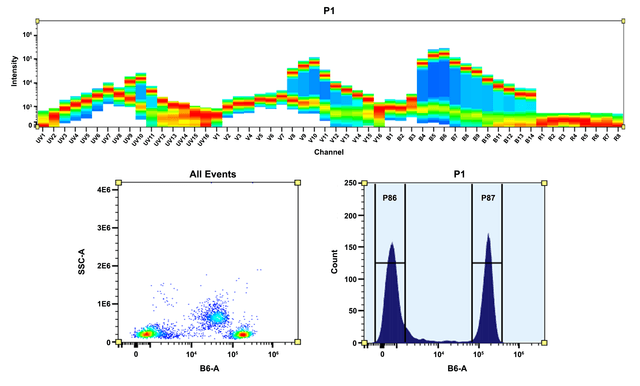
The Degree of Substitution (DOS) is the most important factor for characterizing dye-labeled protein. Proteins of lower DOS usually have weaker fluorescence intensity, but proteins of higher DOS (e.g. DOS > 6) tend to have reduced fluorescence too. The optimal DOS for most antibodies is recommended between 2 and 10 depending on the properties of dye and protein. For effective labeling, the degree of substitution should be controlled to have 6-8 moles of iFluor™ 597 SE to one mole of antibody. The following steps are used to determine the DOS of iFluor™ 597 SE labeled proteins.
Measure absorption
To measure the absorption spectrum of a dye-protein conjugate, it is recommended to keep the sample concentration in the range of 1-10 µM depending on the extinction coefficient of the dye.
Read OD (absorbance) at 280 nm and dye maximum absorption (ƛmax = 597 nm for iFluor™ 597 dyes)
For most spectrophotometers, the sample (from the column fractions) need be diluted with de-ionized water so that the OD values are in the range of 0.1 to 0.9. The O.D. (absorbance) at 280 nm is the maximum absorption of protein while 597 nm is the maximum absorption of iFluor™ 597 SE. To obtain accurate DOS, make sure that the conjugate is free of the non-conjugated dye.
Calculate DOS
You can calculate DOS using our tool by following this link: https://www.aatbio.com/tools/degree-of-labeling-calculator
Spectrum
Product family
| Name | Excitation (nm) | Emission (nm) | Extinction coefficient (cm -1 M -1) | Quantum yield | Correction Factor (260 nm) | Correction Factor (280 nm) |
| iFluor® 350 succinimidyl ester | 345 | 450 | 200001 | 0.951 | 0.83 | 0.23 |
| iFluor® 405 succinimidyl ester | 403 | 427 | 370001 | 0.911 | 0.48 | 0.77 |
| iFluor® 430 succinimidyl ester | 433 | 498 | 400001 | 0.781 | 0.68 | 0.3 |
| iFluor® 440 succinimidyl ester | 434 | 480 | 400001 | 0.671 | 0.352 | 0.229 |
| iFluor® 445 succinimidyl ester | 446 | 558 | - | - | - | - |
| iFluor® 450 succinimidyl ester | 451 | 502 | 400001 | 0.821 | 0.45 | 0.27 |
| iFluor® 460 succinimidyl ester | 468 | 493 | 800001 | ~0.81 | 0.98 | 0.46 |
| iFluor® 488 succinimidyl ester | 491 | 516 | 750001 | 0.91 | 0.21 | 0.11 |
| iFluor® 500 succinimidyl ester | 501 | 520 | 800001 | - | 0.206 | 0.088 |
Show More (36) | ||||||
Citations
Authors: Filep, S and Reid Black, K and Smith, B and Bermingham, M and Pomes, A and Chapman, M
Journal: Clinical Chemistry (2024): hvae106--429
Authors: Sidhu, N and Maksymowych, WP and Wichuk, S and Marotta, A
Journal: Clinical Chemistry (2024): hvae106--427
Authors: Shamsundar, K and Ansari, KI and Bergmann, S and Kleven, K and Soldo, J
Journal: Clinical Chemistry (2024): hvae106--428
References
Authors: Xu, Dongsheng and Zou, Ling and Zhang, Wenjie and Liao, Jieying and Wang, Jia and Cui, Jingjing and Su, Yuxin and Wang, Yuqing and Guo, Yating and Shen, Yi and Bai, Wanzhu
Journal: Frontiers in integrative neuroscience (2021): 728747
Authors: Chang, Gary Ro-Lin and Tu, Min-Yu and Chen, Yu-Hsuan and Chang, Ku-Yi and Chen, Chien-Fu and Lai, Jen-Chieh and Tung, Yu-Tang and Chen, Hsiao-Ling and Fan, Hueng-Chuen and Chen, Chuan-Mu
Journal: Molecular nutrition & food research (2021): e2100182
Authors: Fan, Yuan-Yao and Liu, Chi-Hsien and Wu, An-Lun and Chen, Hung-Chi and Hsueh, Yi-Jen and Chen, Kuan-Jen and Lai, Chi-Chun and Huang, Chung-Ying and Wu, Wei-Chi
Journal: European journal of pharmacology (2021): 174035
Authors: Grünert, Ulrike and Lee, Sammy C S and Kwan, William C and Mundinano, Inaki-Carril and Bourne, James A and Martin, Paul R
Journal: Brain structure & function (2021)
Authors: Shebindu, Adam and Somaweera, Himali and Estlack, Zachary and Kim, Jungtae and Kim, Jungkyu
Journal: Talanta (2021): 122039