iFluor® 510 maleimide
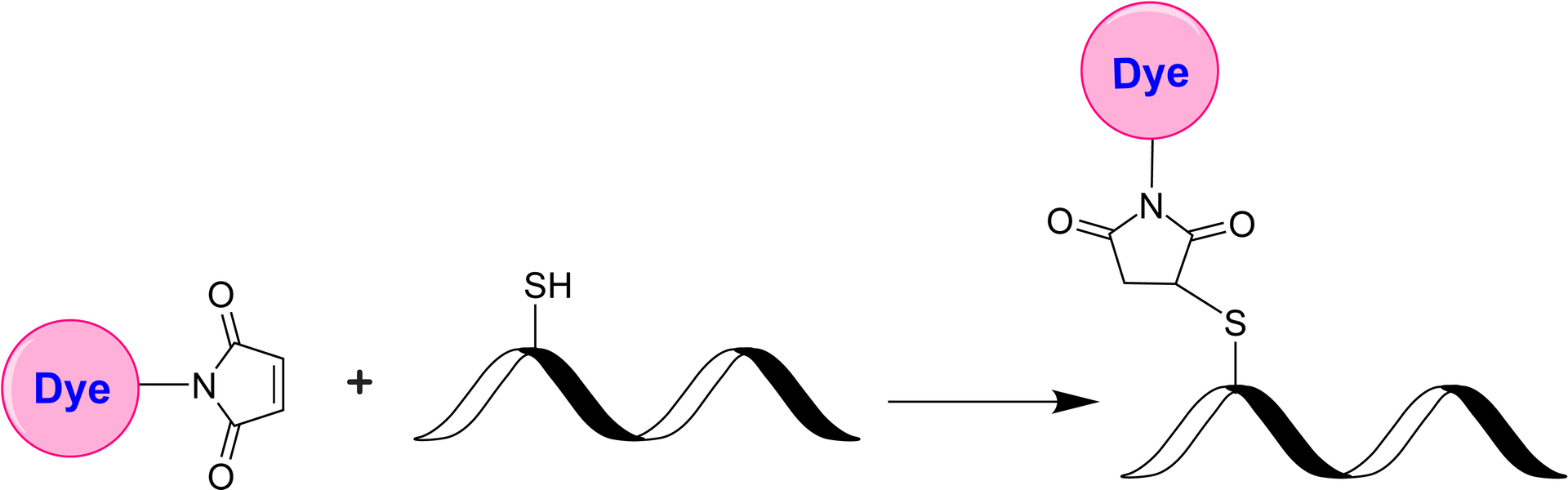
iFluor® 510 maleimide selectively reacts with thiol group of a biomolecule. It is widely used to label the reduced antibodies. iFluor®510 is a new fluorescent dye belonging to the iFluor® family of dyes. These dyes are known for their bright fluorescence, photostability, and compatibility with various imaging techniques and instruments. iFluor® 510 emits green fluorescence when excited with light in the blue to green range (around 488 to 514 nm). Its emission peak is typically around 520 to 530 nm. The number "510" in its name represents the approximate maximum excitation wavelength. Like other iFluor® dyes, AAT Bioquest offers a variety of iFluor® 510 derivatives that can be readily used to develop conjugates for biological research and imaging applications. They can be conjugated to various biomolecules, such as antibodies, proteins, nucleic acids, and small molecules, for fluorescence microscopy, flow cytometry, immunohistochemistry, and other fluorescence-based assays.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 1425 | 1 mg | Price |
Physical properties
| Molecular weight | 875.77 |
| Solvent | DMSO |
Spectral properties
| Excitation (nm) | 511 |
| Emission (nm) | 530 |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| Storage | Freeze (< -15 °C); Minimize light exposure |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on January 16, 2026

