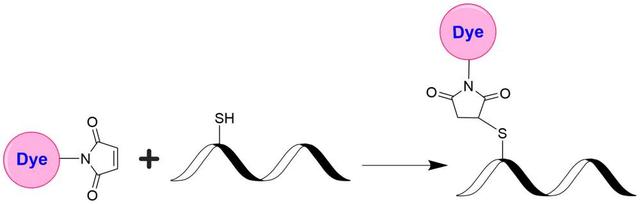
iFluor® 665 maleimide
Example protocol
PREPARATION OF STOCK SOLUTIONS
Unless otherwise noted, all unused stock solutions should be divided into single-use aliquots and stored at -20 °C after preparation. Avoid repeated freeze-thaw cycles
- Allow the vial of iFluor Dye maleimide to warm up to room temperature.
- Add anhydrous DMSO to the vial to prepare a 10 mM dye stock solution.
- Vortex the vial briefly to fully dissolve the dye, and then centrifuge to collect the dye at the bottom of the vial.
- Protect all stock solutions from light as much as possible by wrapping containers in aluminum foil.
- If your protein already contains a thiol group, prepare the protein at 50-100 uM (for example: 5mg/ml BSA is ~75uM) in 50~100 mM MES buffer or buffers of your choice with pH 6.5~7.0.
- If labeling with an intact antibody, reduction of disulfide bonds need to be carried out before maleimide reaction. Prepare antibody in 2-10 mg/ml in a suitable buffer with pH 7.0–7.5. A 10-fold molar excess of a reducing agent such as DTT or TCEP is added to the antibody. If DTT is used, it must be removed by dialysis or desalting to a suitable buffer with pH 6.5~7.0 prior to conjugation. If TCEP is used, it is not necessary to remove excess TCEP during conjugation with maleimides, however, removal of TCEP by dialysis or desalting prior to conjugation gives the better labeling efficiency.
Below is a sample protocol for generating free thiol groups on antibody:-
Prepare 2-10mg/ml IgG solution in PBS.
-
Prepare a fresh solution of 1 M DTT (15.4 mg/100 µL) in distilled water.
-
Add 1- 20 µL of DTT stock per ml of IgG solution while mixing.
-
Let the solution stand at room temperature for 30 minutes without additional mixing (to minimize the re-oxidation of cysteines to cystines).
-
Pass the reduced IgG over a filtration column pre-equilibrated with 50 mM MES buffer (pH=6.5) to remove excess DTT.
-
Determine the antibody concentrations. This can be done either spectrophotometrically or colorimetrically.
-
Carry out the conjugation as soon as possible after this step.
Note: For the best results, IgG solutions should be > 2 mg/mL.
Note: The reduction can be carried out in almost any buffer from pH 7 to 7.5, e.g., MES, phosphate, or TRIS buffers.Note: Steps 5 can be replaced by dialysis.
-
- If your protein doesn’t have a free thiol group or disulfide bond to reduce, a thiolation modification need to be carried out before maleimide conjugation (for example: using 2-Iminothiolane or 2-IT) to introduce sulfhydryl (-SH) groups to the original amino groups on protein.
SAMPLE EXPERIMENTAL PROTOCOL
This labeling protocol was developed for the labeling IgG with iFluor® Dye maleimide. Further optimization may be required for your specific proteins.
Note: Each protein requires a distinct dye/protein ratio, which also depends on the properties of dyes. Over-labeling of a protein could detrimentally affect its binding affinity while the protein conjugates of low dye/protein ratio give reduced sensitivity.
- Use a 10~20:1 molar ratio of iFluor Dye Maleimide dye: IgG as the starting point. While stirring or vortexing the protein solution, add a volume of dye stock solution to result in a dye: protein molar ratio of 10-20. For example, for 5mg/ml IgG (~33 uM), you would add dye to a final concentration of 0.33-0.66 mM.
Note: We recommend using a 10:1 molar ratio of dye to protein. If the ratio is too low or too high, determine the optimal dye/protein ratio at 5:1, 15:1, and 20:1, respectively. - Continue to rotate or shake the reaction mixture at room temperature for 30-60 minutes.
Purify the conjugate on a gel filtration column, such as a Sephadex G-25 column or equivalent matrix, or by extensive dialysis at 4°C in an appropriate buffer.
Recommended AAT Desalting Columns:
| Volume of Reaction | Catalog# |
| 0.6-1.0mL | Cat#60504: PD-10 Column https://www.aatbio.com/products/readiuse-disposable-pd-10-desalting-column?unit=60504 |
| ~0.1mL | Cat#60500: Spin Column https://www.aatbio.com/products/readiuse-bio-gel-p-6-spin-column?unit=60500 |
Determining the Degree of Substitution (DOS) is crucial in characterizing dye-labeled proteins. Lower DOS proteins tend to have weaker fluorescence, but higher DOS proteins may also have reduced fluorescence. For most antibodies, the optimal DOS is between 2 and 10, depending on the dye and protein properties. For effective labeling, the degree of substitution should be controlled to have 5-8 moles of iFluor® 665 maleimide to one mole of antibody. The following steps are used to determine the DOS of iFluor® 665 maleimide-labeled proteins:
- Measure absorption— To measure the absorption spectrum of a dye-protein conjugate, the sample concentration should be kept between 1 and 10 µM (For example: IgG conjugate: 10uM is ~1.5mg/ml), depending on the dye's extinction coefficient.
- Read OD (absorbance) at 280 nm and dye maximum absorption (ƛ max = 667 nm for iFluor® 665 dyes). For most spectrophotometers, the sample (from the column fractions) must be diluted with de-ionized water so that the OD values range from 0.1 to 0.9. The O.D. (absorbance) at 280 nm is the maximum absorption of protein, while 667 nm is the maximum absorption of iFluor® 665 maleimide. To obtain accurate DOS, ensure the conjugate is free of the non-conjugated dye.
- Calculate DOS using our DOS calculator: https://www.aatbio.com/tools/degree-of-labeling-calculator
Spectrum
Product family
| Name | Excitation (nm) | Emission (nm) | Extinction coefficient (cm -1 M -1) | Quantum yield | Correction Factor (260 nm) | Correction Factor (280 nm) |
| iFluor® 350 maleimide | 345 | 450 | 200001 | 0.951 | 0.83 | 0.23 |
| iFluor® 405 maleimide | 403 | 427 | 370001 | 0.911 | 0.48 | 0.77 |
| iFluor® 430 maleimide | 433 | 498 | 400001 | 0.781 | 0.68 | 0.3 |
| iFluor® 450 maleimide | 451 | 502 | 400001 | 0.821 | 0.45 | 0.27 |
| iFluor® 460 maleimide | 468 | 493 | 800001 | ~0.81 | 0.98 | 0.46 |
| iFluor® 488 maleimide | 491 | 516 | 750001 | 0.91 | 0.21 | 0.11 |
| iFluor® 510 maleimide | 511 | 530 | - | - | - | - |
| iFluor® 514 maleimide | 511 | 527 | 750001 | 0.831 | 0.265 | 0.116 |
| iFluor® 532 maleimide | 537 | 560 | 900001 | 0.681 | 0.26 | 0.16 |
Show More (25) | ||||||
References
Authors: Ye, Huamao and Yang, Yue and Chen, Rui and Shi, Xiaolei and Fang, Yu and Yang, Jun and Dong, Yuanzhen and Chen, Lili and Xia, Jianghua and Wang, Chao and Yang, Chenghua and Feng, Jun and Wang, Yang and Feng, Xiang and Lü, Chen
Journal: Current pharmaceutical design (2020): 1614-1621
Authors: Roobsoong, Wanlapa and Maher, Steven P and Rachaphaew, Nattawan and Barnes, Samantha J and Williamson, Kim C and Sattabongkot, Jetsumon and Adams, John H
Journal: Malaria journal (2014): 55
Authors: Dutta, Palash K and Lin, Su and Loskutov, Andrey and Levenberg, Symon and Jun, Daniel and Saer, Rafael and Beatty, J Thomas and Liu, Yan and Yan, Hao and Woodbury, Neal W
Journal: Journal of the American Chemical Society (2014): 4599-604
Authors: Maawy, Ali A and Hiroshima, Yukihiko and Kaushal, Sharmeela and Luiken, George A and Hoffman, Robert M and Bouvet, Michael
Journal: Journal of biomedical optics (2013): 126016
Authors: Chou, Cheng-Chung and Huang, Yi-Han
Journal: Sensors (Basel, Switzerland) (2012): 16660-72